|
Our lab is investigating the use of novel materials, like cubic silicon carbide (3C-SiC), to produce a long-term (75+ years), bi-directional, implantable neural prosthetic for brain machine interface systems. The NLML has a multdisciplanary group working to achieve this goal. Dr. Stephen Saddow has pioneered the use of 3C-SiC as a biocompatible material, has extensive experience the growth and processing 3C-SiC, and is known internationally as a quality leader. His former graduate student, Chris Frewin, Ph.D., has joined the NLML as a postdoctoral fellow. Our partner, Dr. Iyad Obeid from Temple University, brings extensive knowledge and experience in neural instrumentation and techniques to interpret the signals from the brain.
Damage to the CNS and PNS caused by disease or trauma reduces the ability to process sensory information, control muscles and hinders learning and memory. The ancient Greeks were among the earliest to employ exogenous electrical impulses as a therapy (electrical impulses generated from fish were used to manage pain) and in the 18th century, the idea of generating the electrical signal artificially by a man-made device was pioneered by Benjamin Franklin. Our understanding of electrical activity and the materials that support that activity has lead to the electronic/ computer age, and the devices used for CNS therapeutics have also benefited from these advances resulting in the brain machine interface (BMI) system.
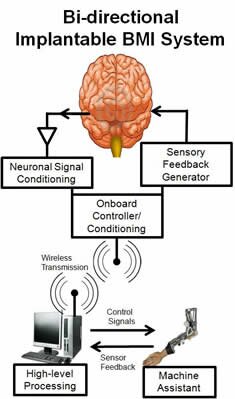
The BMI system uses an interface to receive electrical signals from the body, transfers the signals to a processing system which then can control the activities of electronics or a machine actuator. Optimally, there would be a method of generating or receiving feedback from the environment and delivering this information back into the brain.The system is illustrated below.
Current BMI systems have shown success in providing relief for some CNS and PNS problems, and have restored hearing, sight, and allowed mental control of limb prosthetics. Unfortunately, widespread utility has been hampered by challenges associated with the neuronal interface of the BMI.
Non-invasive external receivers have many difficulties steaming from large impedance from the skin and skull, crosstalk, noise, and low specificity. The alternative is using an implantable neuronal prosthetic which allows direct interaction with specific neurons within the brain. This direct contact not only increases signal selectivity, but allows controlled stimulation of the neurons and thereby creates bi-directional signaling.
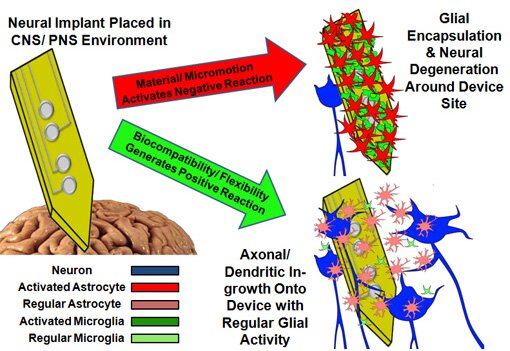
Apart from the surgery that is required to implant these interface devices, they eventually stop receiving signals in vivo after only a few years. As shown in the cartoon below, much of the device failure has been attributed to increased negative activity from the glia (support/ immune) cells as well as decreased axon/ dendritic activity and population of nearby neurons. The potential of BMI devices is limited currently due to the failure of the implantable neural interface.
Our current efforts are as follows:
 
This work is funded by:
A grant from the USF Neuroscience Collaborative (NSC),
National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA) to C. Frewin, and
A grant from the Florida Center of Excellence for Biomolecular Identification and Targeted Therapeutics (Now CDDI).
|

