|
The UBE3A gene product was discovered while studying the role of p53 tumor suppressor protein degradation by the oncogenic human papilloma virus (HPV). Studies involving HPV E6 gene expression following viral infection found that the HPV E6 protein needed an associated protein for p53 degradation (15-18). This associated protein was defined as an E6-associated protein (E6-AP); a 100 kDa protein that functioned as an ubiquitin ligase enzyme targeting the p53 for degradation through the proteosomal pathway. Multiple steps are involved in the ubiquitination process (Figure below). In the initial enzymatic step, the ubiquitin activating enzyme E1 activates ubiquitin in an ATP-dependent reaction that links the E1 with the C-terminus of an ubiquitin molecule. The ubiquitin conjugating E2 enzymes then accept and transfer the activated ubiquitin moiety from E1 either to an E3 ligase or directly to a target substrate. The E3 ligase confers specificity to substrate recognition for ubiquitination. This is sometimes associated with an E4 ligase that will add a specific number of ubiquitin molecules to the chain to create a length that then signals for proteosomal degradation. The underlying cause of AS is the absence of the UBE3A gene that codes for E6-AP (19, 20). At the time of its discovery, the general belief was that specific protein targets of Ube3a were accumulating and thus resulting in generalized synaptic dysfunction. Moreover, these protein targets in the human CNS would be relatively easy to identify due to the fact that Ube3a falls into the E3 category of ubiquitin ligases and should have a limited number of targeted proteins. However, the quest to find Ube3a targets has proven a difficult one.
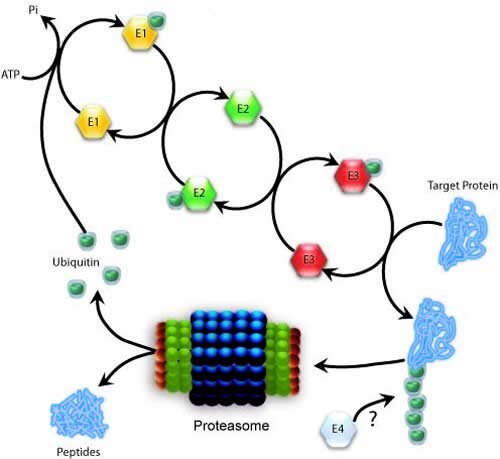
Using traditional methods of identifying ubiquinated proteins through 2-D gel electrophoresis, a number of proteins were identified as targets of Ube3a including the P53 tumor suppressor protein, the HHR23A protein; a human homologue to the yeast DNA repair protein Rad23 (21) and the multicopy maintenance protein (Mcm) 7 subunit involved in the initiation of DNA replication (22). Interestingly, E6-AP was found to be a target for itself, suggesting a regulation of protein concentration at the biochemical level in addition to control over allelic expression at the genetic level through genetic imprinting (23). The number of targets was recently expanded to include the protein Arc (24). The Arc protein is found enriched in synapses and has at least one role in regulating the surface expression of AMPA-type receptors (AMPAR). Thus, in the absence of Ube3a, Arc protein accumulates and reduces the surface complement of AMPAR. This reduction in AMPAR results in a reduction in baseline synaptic transmission and decreased overall AMPAR function and sensitivity.
Current NLML AS Research Projects
Introduction to Angelman Syndrome
Model Systems of Angelman Syndrome
Molecular Changes in the AS Mouse Model
Parents Area
15. J. A. Mietz, T. Unger, J. M. Huibregtse, P. M. Howley, Embo J 11, 5013 (1992).
16. S. Waddell, J. R. Jenkins, Oncogene 16, 1759 (1998).
17. J. S. Park et al., Gynecol Oncol 65, 121 (1997).
18. C. C. Harris, J Investig Dermatol Symp Proc 1, 115 (1996).
19. J. M. Huibregtse, M. Scheffner, P. M. Howley, Embo J 10, 4129 (1991).
20. J. M. Huibregtse, M. Scheffner, P. M. Howley, Mol Cell Biol 13, 775 (1993).
|

